Ablation procedures demand precision, efficiency, and a well-organized clinical environment. Whether used in electrophysiology, cardiac care, or oncology, these treatments rely on specialized equipment and streamlined workflows.
A purpose-built ablation cart plays a critical role in supporting this process—offering secure storage, mobility, and integration for ablation systems and accessories.
For OEM device manufacturers, developing a medical cart for ablation devices means designing and developing a product that not only meets clinical performance standards such as IEC 60601-1 but also enhances the user experience for clinicians and biomedical teams.
Key Design Considerations for Ablation Carts
When developing a medical cart for ablation devices, precision in design and clinical functionality are essential. Each feature should contribute to efficiency, durability, and ease of use.
-
Durable, Medical-Grade Construction

Ablation carts must withstand frequent cleaning and disinfection. High-grade materials such as powder-coated steel or antimicrobial polymers ensure longevity and compliance with healthcare hygiene standards. -
 Customizable Drawer and Shelf Configurations
Customizable Drawer and Shelf Configurations
Different ablation systems require unique setups. Modular designs allow device manufacturers to tailor their drawer sizes, shelf heights, and storage layouts to accommodate the specific needs of the hospitals or care facilities they serve . -
 Cable and Power Management
Cable and Power Management
Effective cable routing minimizes clutter, enhances safety, and improves workflow. Integrated power options—such as outlets, cable wraps, and device docking—ensure that ablation generators and monitors are always connected and ready for use. -
 Mobility and Ergonomics
Mobility and Ergonomics
Smooth-rolling casters, locking mechanisms, and lightweight frames help clinicians move equipment safely between operating rooms. Adjustable heights and ergonomic handles reduce strain during frequent repositioning. -
 Equipment Integration and Connectivity
Equipment Integration and Connectivity
Modern ablation carts often include mounts or brackets for monitors and data interfaces, enabling clinicians to view real-time ablation data directly from the workstation. Manufacturers should consider future-proofing designs with adaptable ports or modular mounts for evolving device technologies.
Ablation Carts vs Other Medical Procedure Carts
While they share similarities with anesthesia or utility carts, ablation carts are uniquely engineered for precision-based procedures. They feature specialized compartments for electrophysiology components, reinforced tops for heavy devices, and enhanced electrical integration.
In ablation procedures, seconds count—so organization and accessibility are critical. A properly designed ablation cart helps clinicians focus on patient care rather than equipment logistics.
Customization: Meeting Facility-Specific Needs
Every healthcare environment is different. Electrophysiology labs, surgical suites, and cardiac centers each have specific workflows and space constraints. Offering custom ablation cart configurations offers the ability to tailor storage, dimensions, and finishes to meet the needs of the device manufacturer or facility.
Examples include:
- Additional drawers for catheters and accessories
- Shelving designed for RF generators or cooling systems
- Integrated monitor arms for visualization equipment
- Lockable compartments for controlled device storage
By collaborating directly with clinicians and biomedical engineers during development, manufacturers can ensure each ablation cart design supports real-world usability and compliance.
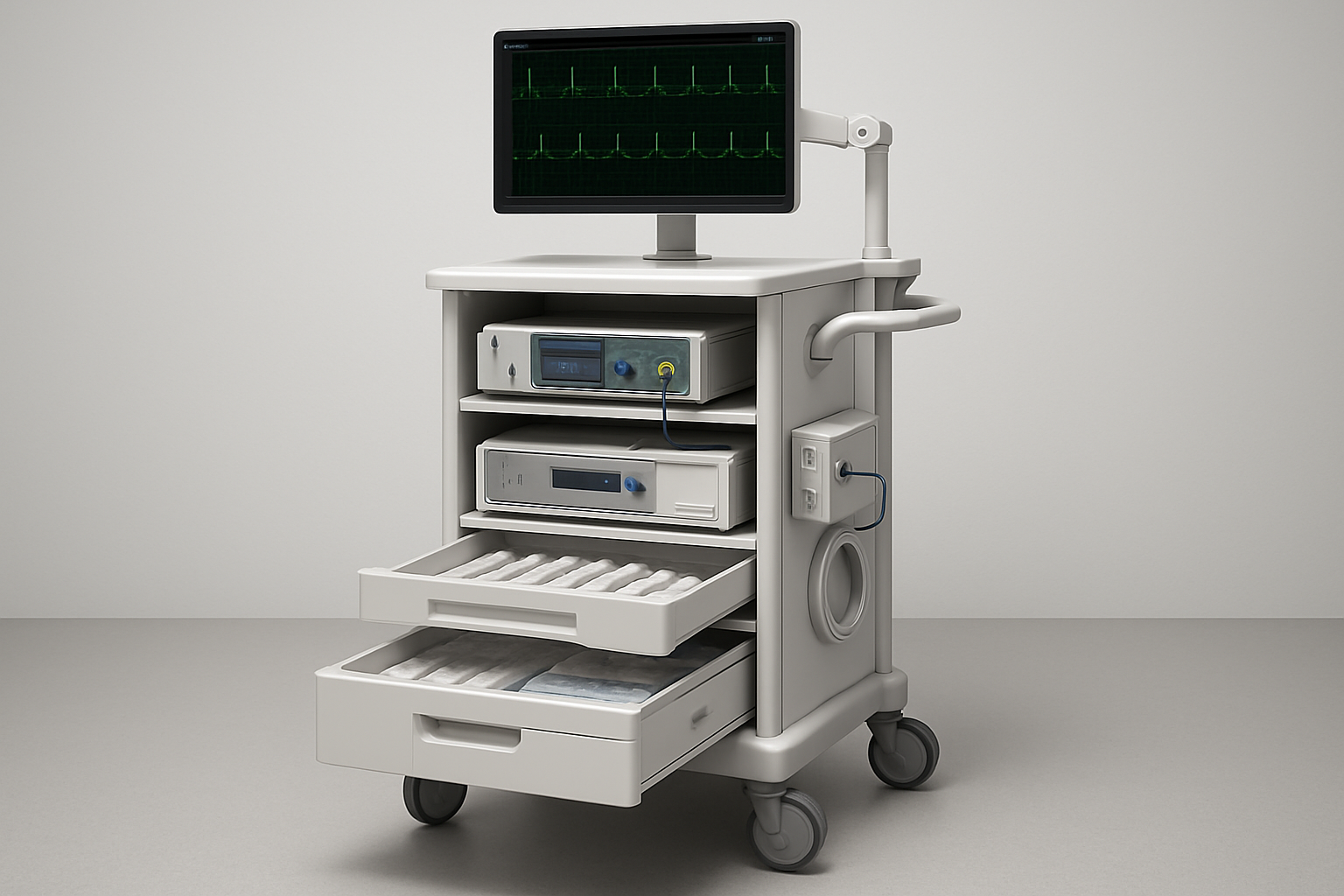
HUI Cart example: SoniVie (Boston Scientific) TIVUS™ Renal Denervation System Console (see pictured)
Developing Ablation Carts
Developing a medical cart for ablation devices requires an understanding of both engineering excellence and clinical workflow. A well-designed ablation cart enhances efficiency, supports safety, and helps clinicians perform with confidence. Because ablation technologies often involve power management, cooling components, and sensitive electronics, the supporting cart must be designed to meet critical IEC 60601-1 requirements.
Partnering with a manufacturer that offers engineering and development services, such as HUI Medical Carts, ensures that IEC 60601-1 considerations are built into the design from the start.
The ablation cart development process may also include preliminary 60601-1 mechanical tests to ensure your cart is safe for clinical use and aligned to pass critical tests for the IEC 60601-1 standard.
As ablation technologies evolve, the right cart design will continue to play a vital role in enabling precision care.